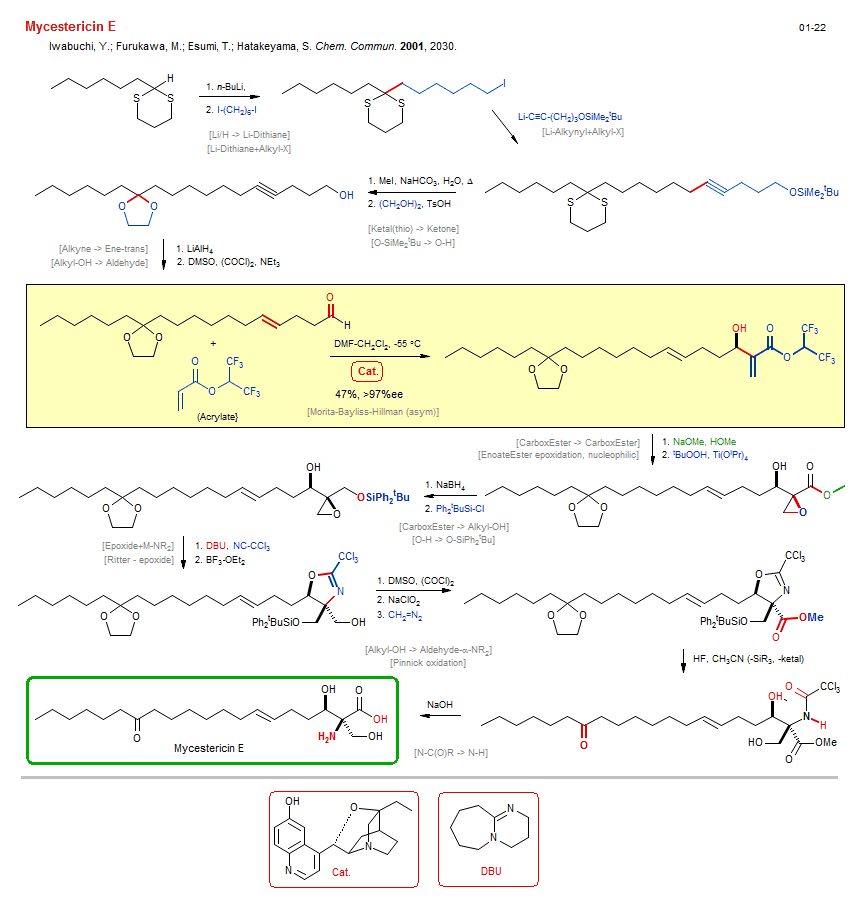
Total synthesis of Mycestericin E:
| Reference: | Iwabuchi, Y.; Furukawa, M.; Esumi, T.; Hatakeyama, S. Chem. Commun. 2001, 2030. DOI |
| Reactions: | O-SiPh2tBu → O-H • Ketal → Ketone • Ritter - epoxide • Epoxide+M-NR2 • Oxazoline • Li/H → Li-Alkynyl • Ketone → Ketal • Li-Dithiane+Alkyl-X • Alkyl-X+Li-R • Li-Alkynyl+Alkyl-X • Alkyl-X+Li-R • Alkyne → Ene-trans • Hydroalumination Alkyne • Morita-Bayliss-Hillman (asym) • Aldol-Base → EnoateEster • Alkyl-OH → Aldehyde • CarboxEster → CarboxEster • EnoateEster epoxidation, nucleophilic • Alkyl-OH → Aldehyde-α-NR2 • Pinnick oxidation • Aldehyde → CarboxAcid • CarboxEster → Alkyl-OH • O-H → O-SiPh2tBu • CompNu-O-H/O-H+R3Si-X • N-C(O)R → N-H • Li/H → Li-Dithiane • Ketal(thio) → Ketone • O-SiMe2tBu → O-H • |
| Reagents: | NaOMe • Hydroperoxide, tbutyl - Ti • BH4-Na+ • SiPh2tBu-Cl • DBU • DMSO, (COCl)2 (Swern) • Chlorite, sodium • Diazomethane • BF3·OEt2 • HF • Hydroxide, sodium • Acetonitrile, trichloro • BuLi(n) • Alkynyl-Li • MeI • Glycol • AlH4-Li+ • DMSO, (COCl)2 (Swern) • DMF (solvent) • Acrylate • |