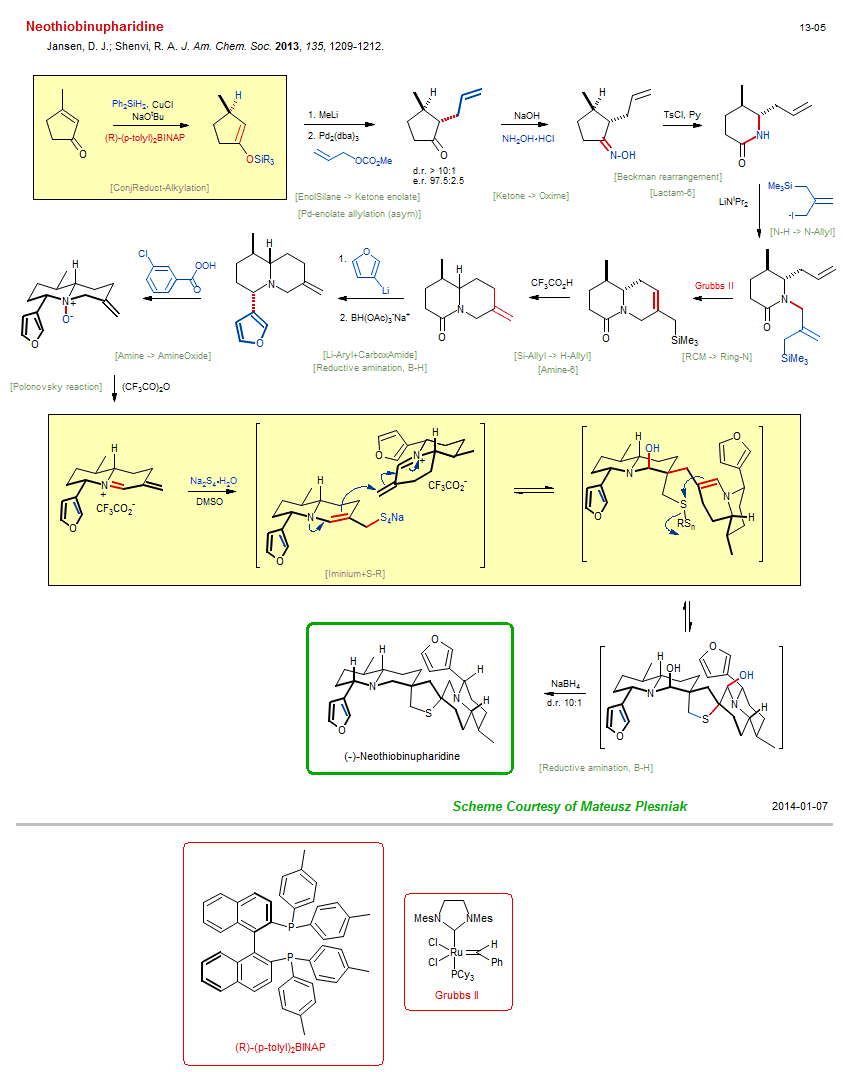
Total synthesis of Neothiobinupharidine:
| Reference: | Jansen, D. J.; Shenvi, R. A. J. Am. Chem. Soc. 2013, 135, 1209-1212. DOI |
| Reactions: | EnolSilane → Ketone enolate • ConjReduct-Alkylation • Pd-enolate allylation (asym) • Tsuji-Trost allylation • Beckman rearrangement • RCM → Ring-N • Bicyclo(440)-aza • Amine → AmineOxide • CompOx-Amine/Alkene+Peracid • Polonovsky reaction • N-H → N-Allyl • Si-Allyl → H-Allyl • Iminium+S-R • Reductive amination, B-H • Spiro(54)-aza • Spiro(54)-thia • Lactam-6 • Amine-6 • Li-Aryl+CarboxAmide • CarboxAmide+Li-R • Furan synthesis • Reductive amination, B-H • EnolSilane → Ketone enolate • Ketone → Oxime • |
| Reagents: | HSiPh2H • NaOtBu • Me-Li • Allyl-OAc • Hydroxide, sodium • NH2OH • TsCl, Py • CuCl • Pd2(dba)3 • BINAP • Allyl-I • Allyl-SiR3 • LiNiPr2 • Grubbs II • Acetic acid, trifluoro • Furyl-Li • BH(OAc)3-Na+ • Acetic anhydride, trifluoro • Sulfide, sodium • BH4-Na+ • DMSO (solvent) • Perbenzoic acid, 3-chloro • |