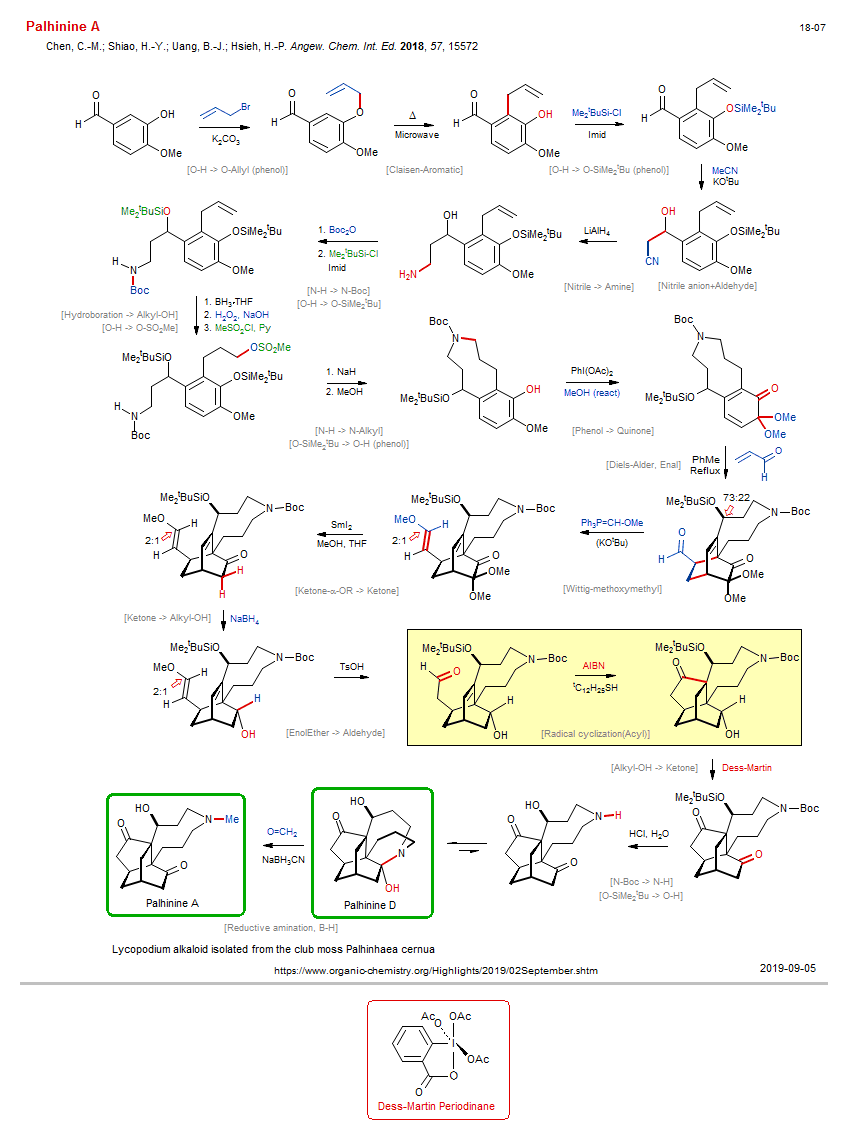
Total synthesis of Palhinine A New:
| Reference: | Chen, C.-M.; Shiao, H.-Y.; Uang, B.-J.; Hsieh, H.-P. Angew. Chem. Int. Ed. 2018, 57, 15572 DOI |
| Reactions: | O-H → O-Allyl (phenol) • Williamson etherification • Claisen-Aromatic • O-H → O-SiMe2tBu (phenol) • Nitrile anion+Aldehyde • Nitrile → Amine • N-H → N-Boc • O-H → O-SiMe2tBu • N-H → N-Alkyl • Amine-9+ • Phenol → Quinone • Diels-Alder, Enal • Bicyclo(222) • Wittig-methoxymethyl • Ketone-α-OR → Ketone • Ketone → Alkyl-OH • EnolEther → Aldehyde • Radical cyclization(Acyl) • Cyclopentanone • Bicyclo(321) • Alkyl-OH → Ketone • N-Boc → N-H • O-SiMe2tBu → O-H • Reductive amination, B-H • Eschweiler-Clarke methylation • Hydroboration → Alkyl-OH • O-H → O-SO2Me • CompNu-O-H/N-H+RSO2X • O-SiMe2tBu → O-H (phenol) • CompE+-Si-O/Si-O+H2O • |
| Reagents: | Allyl-Br • Carbonate, potassium • Microwave • SiMe2tBu-Cl • Acetonitrile (react) • KOtBu • AlH4-Li+ • Boc2O • SiMe2tBu-Cl • BH3·THF • H2O2, NaOH • Sulfonyl chloride, methane, Py • NaH • Iodosobenzene-OAc • HOMe (react) • Acrolein • PPh3=CH-OR • KOtBu • SmI2 • BH4-Na+ • TsOH • AIBN • Dess-Martin • HCl, H2O • Formaldehyde • BH3CN-Na+ • |