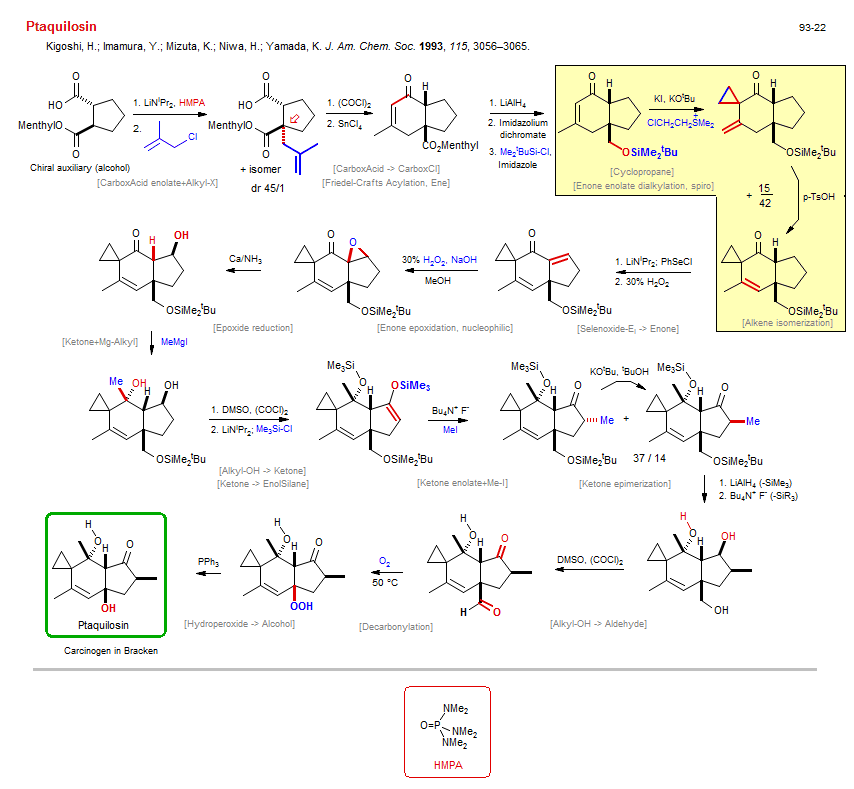
Total synthesis of Ptaquilosin:
| Reference: | Kigoshi, H.; Imamura, Y.; Mizuta, K.; Niwa, H.; Yamada, K. J. Am. Chem. Soc. 1993, 115, 3056–3065. DOI |
| Reactions: | CarboxEster → Alkyl-OH • Allyl-OH → Enone • O-H → O-SiMe2tBu • Ketone → Ketone-α-SeR • Selenide → Selenoxide • EnolSilane → Ketone enolate • O-SiMe3 → O-H • O-SiMe2tBu → O-H • Friedel-Crafts Acylation, Ene • Cyclohexenone • Bicyclo(430) • Ketone enolate+Me-I • CompE+-Si-O/Si-O+M-R • Fluorodesilylation → Enolate • Selenoxide-Ei → Enone • Ketone → Enone • Enone enolate dialkylation, spiro • Deconjugative alkylation • Alkyl-X+Enolate (intram) • Enone enolate+Alkyl-X • CarboxAcid enolate+Alkyl-X • Allyl-X+Enolate (asym) • Enone epoxidation, nucleophilic • CompOx-Enone/Alkene+ROOH • Ketone+Mg-Alkyl • Mg-Alkyl+Ketone • Ketone → Ketone-α-SeR • Epoxide reduction • Decarbonylation • C-C(O)H → C-OH • Hydroperoxide → Alcohol • Cyclopropane • Spiro(52) • Alkene isomerization • Ketone epimerization • CarboxAcid → CarboxCl • Alkyl-OH → Ketone • CompOx-Alkyl-OH/Alkyl-OH+DMSO • Ketone → EnolSilane • Alkyl-OH → Aldehyde • |
| Reagents: | LiNiPr2, HMPA • Allyl-Cl • Oxalyl chloride • SnCl4 • AlH4-Li+ • Cr2O7·2ImH • SiMe2tBu-Cl • Ethylene dichloride • KOtBu • LiNiPr2 • H2O2 • H2O2, NaOH • Ca°/NH3 • Me-MgI • DMSO, (COCl)2 (Swern) • LiNiPr2 • Fluoride, R4N+ • MeI • AlH4-Li+ • DMSO, (COCl)2 (Swern) • Fluoride, R4N+ • O2 • PPh3 • SePh-Cl • SiMe3-Cl • Imidazole • KOtBu • Chiral auxiliary (alcohol) • TsOH • |