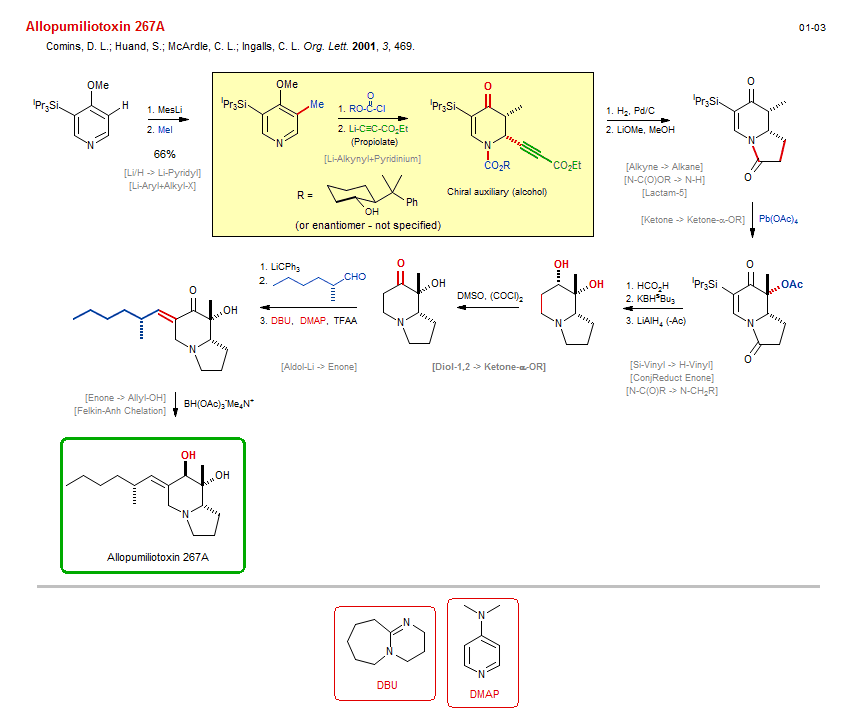
Total synthesis of Allopumiliotoxin 267A:
| Reference: | Comins, D. L.; Huand, S.; McArdle, C. L.; Ingalls, C. L. Org. Lett. 2001, 3, 469. DOI |
| Reactions: | Aldol-Li → Enone • Ketone enolate+Aldehyde • Alkyne → Alkane • Lactam-5 • O-C(O)R → O-H • N-C(O)R → N-CH2R • Amine-5 • Felkin-Anh Chelation • Li/H → Li-Pyridyl • Ketone → Ketone-α-OR • Bicyclo(430)-aza • Li-Alkynyl+Pyridinium • Pyridinium+M-R • Li/H → Li-Alkynyl • Diol-1,2 → Ketone-α-OR • CompOx-Alkyl-OH/Amine+DMSO • Li-Aryl+Alkyl-X • Alkyl-X+Li-R • Lactam-5 • Si-Vinyl → H-Vinyl • Enone → Allyl-OH • ConjReduct Enone • N-C(O)OR → N-H • |
| Reagents: | Mesityl-Li • MeI • H2, Pd • LiOMe • Pb(OAc)4 • Formic acid • BH(sBu3)-K+ • DMSO, (COCl)2 (Swern) • LiCPh3 • DBU • BH(OAc)3-Me4N+ • Acetic anhydride, trifluoro • AlH4-Li+ • DMAP • O=C(OR)Cl • Alkynyl-Li • Propiolate • Chiral auxiliary (alcohol) • |