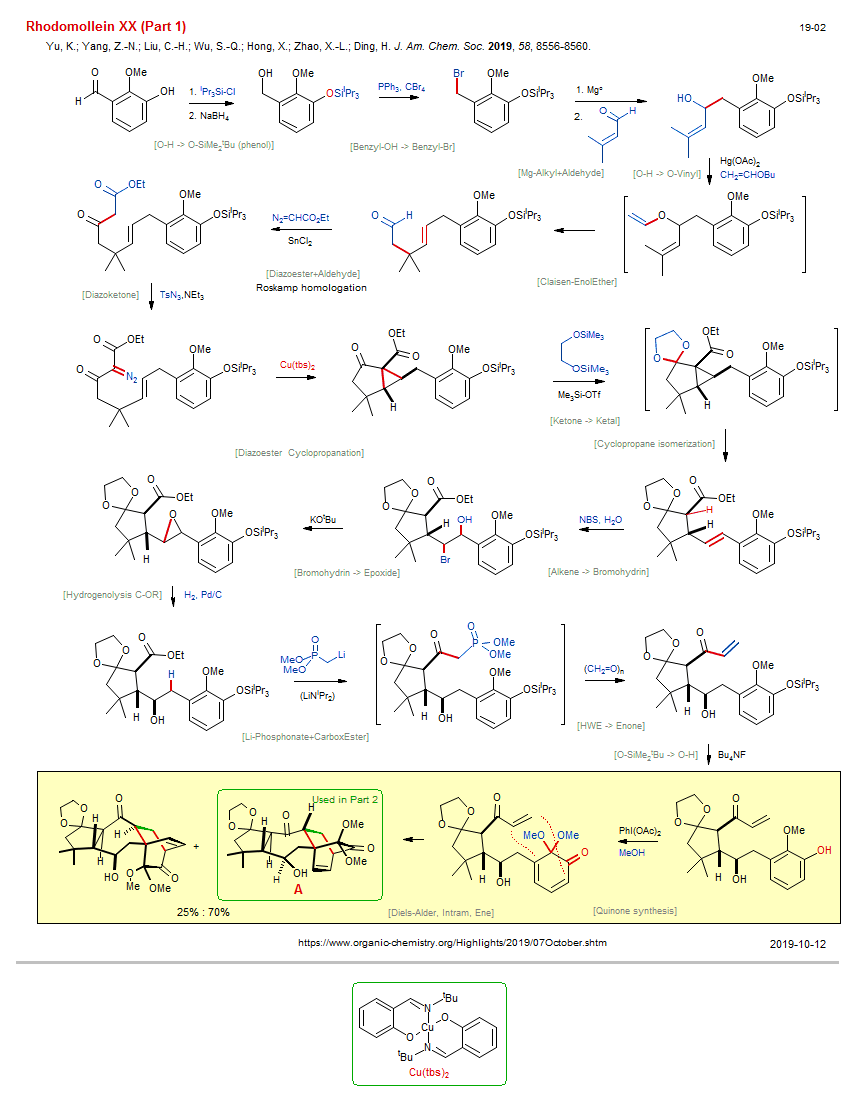
Total synthesis of Rhodomollein XX (Part 1) New:
| Reference: | Yu, K.; Yang, Z.-N.; Liu, C.-H.; Wu, S.-Q.; Hong, X.; Zhao, X.-L.; Ding, H. J. Am. Chem. Soc. 2019, 58, 8556-8560. DOI (Part 2) |
| Reactions: | O-H → O-SiMe2tBu (phenol) • Benzyl-OH → Benzyl-Br • Appel-Br • Mg-Alkyl+Aldehyde • Aldehyde+Mg-R • O-H → O-Vinyl • Claisen-EnolEther • Diazoester+Aldehyde • Diazoketone • DiazoEster • Diazoester Cyclopropanation • Cyclopropane • Bicyclo(310) • Ketone → Ketal • Alkene → Bromohydrin • Bromohydrin → Epoxide • Epoxidation via halohydrin • Hydrogenolysis C-OR • Epoxide reduction • Li-Phosphonate+CarboxEster • HWE → Enone • O-SiMe2tBu → O-H • Quinone synthesis • Ketone → Ketal • Diels-Alder, Intram, Ene • Diels-Alder, Quinone • Bicyclo(222) • Bicyclo(430) • Cyclopropane isomerization • |
| Reagents: | SiiPr3-Cl • BH4-Na+ • PPh3, CBr4 • Mg° • Hg(OAc)2 • Diazoacetate • SnCl2 • Azide, sulfonyl • NEt3 • SiMe3-OTf • Glycol, bistrimethylsilylether • NBS • KOtBu • H2, Pd • LiCH2-PO(OMe)2 • Formaldehyde • Fluoride, R4N+ • Iodosobenzene-OAc • HOMe (react) • LiNiPr2 • |