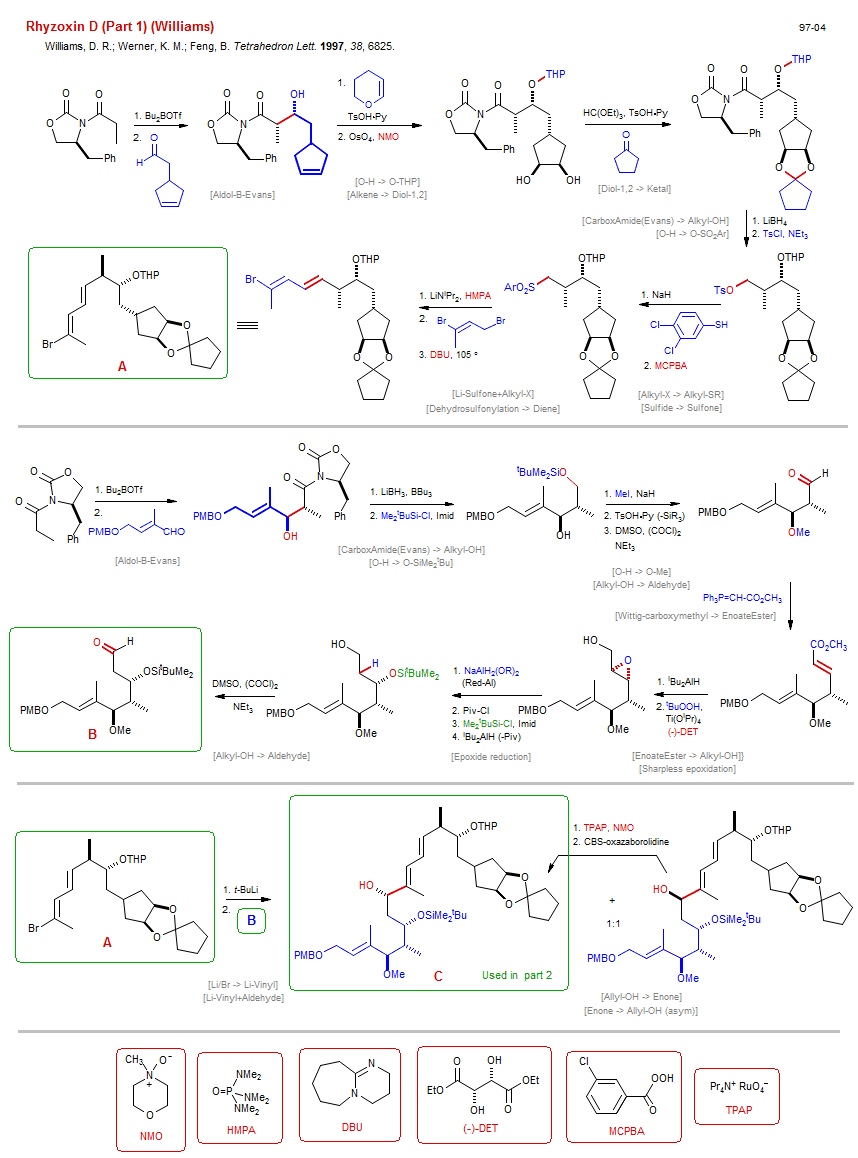
Total synthesis of Rhyzoxin D (Part 1) (Williams):
| Reference: | Williams, D. R.; Werner, K. M.; Feng, B. Tetrahedron Lett. 1997, 38, 6825. DOI (Part 2) |
| Reactions: | Li/H → Li-Sulfone • Aldol-B-Evans • CarboxAmide enolate+Aldehyde (asym) • Wittig-carboxymethyl → EnoateEster • Li-Sulfone+Alkyl-X • Allyl-X+Li-R • Li/Br → Li-Vinyl • Dehydrosulfonylation → Diene • Aldol-B-Evans • CarboxAmide enolate+Aldehyde (asym) • Li-Vinyl+Aldehyde • O-SiMe2tBu → O-H • O-H → O-Piv • O-H → O-SiMe2tBu • O-Piv → O-H • O-H → O-THP • Alkene → Diol-1,2 • Diol-1,2 → Ketal • CarboxAmide(Evans) → Alkyl-OH • O-H → O-SO2Ar • Alkyl-X → Alkyl-SR • Sulfide → Sulfone • CarboxAmide(Evans) → Alkyl-OH • O-H → O-SiMe2tBu • O-H → O-Me • Alkyl-OH → Aldehyde • EnoateEster → Alkyl-OH • Sharpless epoxidation • Epoxide reduction • Alkyl-OH → Aldehyde • Allyl-OH → Enone • Enone → Allyl-OH (asym) • |
| Reagents: | B(OTf)Bu2 • LiNiPr2, HMPA • DBU • B(OTf)Bu2 • PPh3=CH-CO2R • BuLi(tert) • Chiral auxiliary (amide-Evans) • LiCHR-Sulfonyl • Chiral auxiliary (amide-Evans) • HMPA (solvent) • Vinyl-Br • Allyl-Br • OsO4 • Orthoformate • BH4-Li+ • TsCl, NEt3 • Thiol, aryl • NaH • Perbenzoic acid, 3-chloro • SiMe2tBu-Cl • MeI • DMSO, (COCl)2 (Swern) • NEt3 • AlHiBu2 • Hydroperoxide, tbutyl • TsOH·Py • AlH2(OR)2-Na+ • Piv-Cl • SiMe2tBu-Cl • AlHiBu2 • DMSO, (COCl)2 (Swern) • NEt3 • Vinyl-Li • Vinyl-Li • RuO4-Pr4N+, NMO • AlH2(OR)2-Na+ • Tartrate, diethyl • DHP • TsOH·Py • |