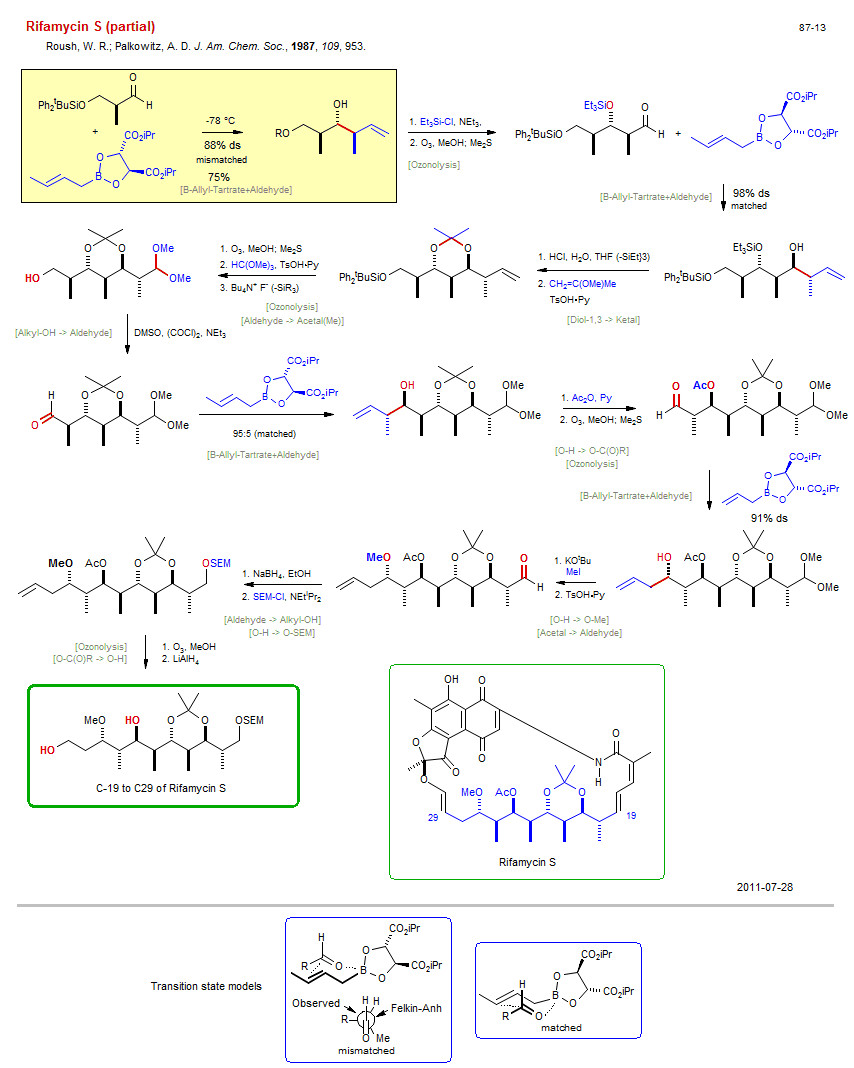
Total synthesis of Rifamycin S (partial):
| Reference: | Roush, W. R.; Palkowitz, A. D. J. Am. Chem. Soc., 1987, 109, 953. DOI |
| Reactions: | O-H → O-SiEt3 • O-SiEt3 → O-H • B-Allyl-Tartrate+Aldehyde • Aldehyde+B-Allyl (asym) • Aldol-B-Tartrate • B-Allyl-Tartrate+Aldehyde • Aldehyde+B-Allyl (asym) • B-Allyl-Tartrate+Aldehyde • Aldehyde+B-Allyl (asym) • Aldol-B-Tartrate • B-Allyl-Tartrate+Aldehyde • Aldehyde+B-Allyl (asym) • Ozonolysis • Diol-1,3 → Ketal • Ozonolysis • Aldehyde → Acetal(Me) • Alkyl-OH → Aldehyde • O-H → O-C(O)R • Ozonolysis • O-C(O)R → O-H • Ozonolysis • O-H → O-Me • Acetal → Aldehyde • CompNu-Acetal/Ketal+H+ • Aldehyde → Alkyl-OH • O-H → O-SEM • |
| Reagents: | Crotyl-B(tartrate) • Chiral auxiliary (boronate) • SiEt3-Cl • O3 • Crotyl-B(tartrate) • Chiral auxiliary (boronate) • Allyl-B(tartrate) • Chiral auxiliary (boronate) • HCl, H2O • Propenyl-OR • O3 • TsOH·Py • Orthoformate • Fluoride, R4N+ • DMSO, (COCl)2 (Swern) • Crotyl-B(tartrate) • Chiral auxiliary (boronate) • Acetic anhydride, Py • O3 • BH4-Na+ • NEtiPr2 • O3 • AlH4-Li+ • KOtBu • MeI • TsOH·Py • |