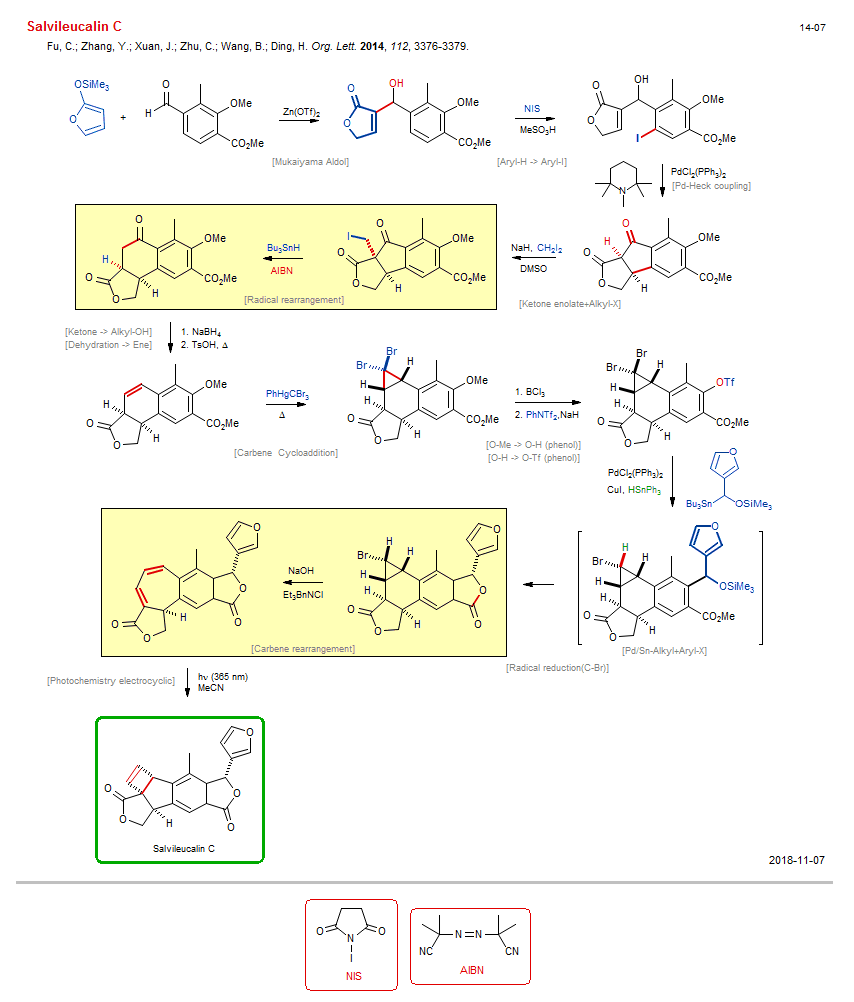
Total synthesis of Salvileucalin C:
| Reference: | Fu, C.; Zhang, Y.; Xuan, J.; Zhu, C.; Wang, B.; Ding, H. Org. Lett. 2014, 112, 3376-3379. DOI |
| Reactions: | Pd-Heck coupling • Cyclopentanone • Mukaiyama Aldol • Aldol-Si • Ketene acetal+Aldehyde • Lactone-5 • Aryl-H → Aryl-I • Ketone enolate+Alkyl-X • Alkyl-X+Enolate • Radical rearrangement • Cyclohexanone • Ketone → Alkyl-OH • Dehydration → Ene • Carbene Cycloaddition • Cyclopropane • Bicyclo(410) • O-Me → O-H (phenol) • O-H → O-Tf (phenol) • Pd/Sn-Alkyl+Aryl-X • Radical reduction(C-Br) • Alkyl-X → Alkyl-H(2°) • Carbene rearrangement • Cycloheptane • Bicyclo(440) • Photochemistry electrocyclic • Electrocyclic-4π • Cyclobutane • Bicyclo(320) • |
| Reagents: | Zn(OTf)2 • NIS • Sulfonic acid, methane • PdCl2(PPh3)2 • NaH • CH2I2 • HSnBu3 • AIBN • DMSO (solvent) • BH4-Na+ • TsOH • PhHgCBr3 • B(Cl)3 • Tf2NPh • NaH • PdCl2(PPh3)2 • CuI • HSnPh3 • Alkyl-SnR3 • Hydroxide, sodium • Chloride, R4N+ • Photochemistry • Acetonitrile (solvent) • |