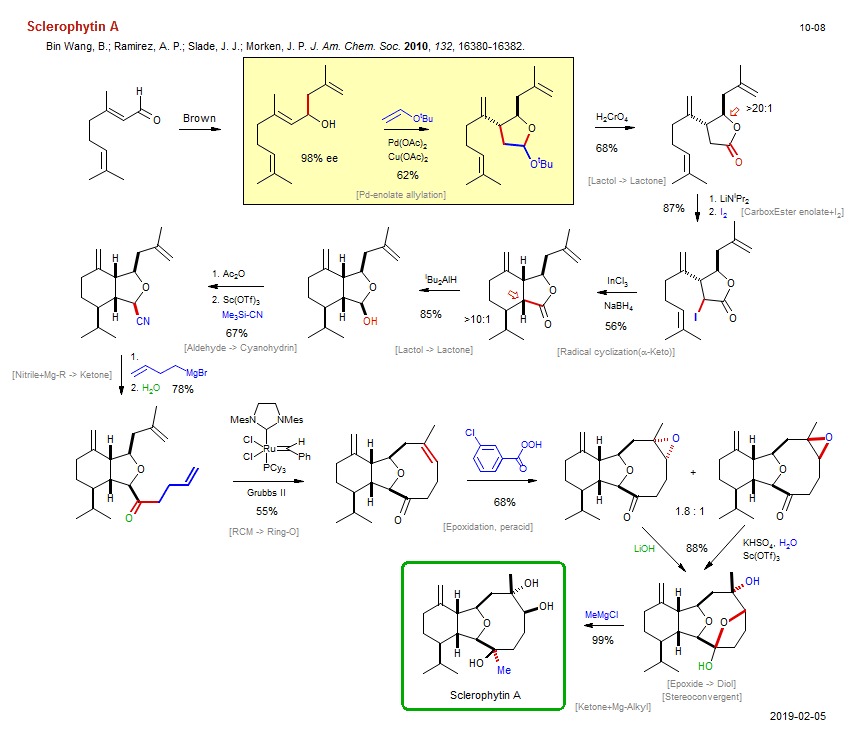
Total synthesis of Sclerophytin A:
| Reference: | Bin Wang, B.; Ramirez, A. P.; Slade, J. J.; Morken, J. P. J. Am. Chem. Soc. 2010, 132, 16380-16382. DOI |
| Reactions: | Pd-enolate allylation • Ether-5 • Lactol → Lactone • Lactone-5 • CarboxEster enolate+I2 • Radical cyclization(α-Keto) • Cyclohexane • Bicyclo(430)-oxa • Lactol → Lactone • Aldehyde → Cyanohydrin • Nitrile+Mg-R → Ketone • RCM → Ring-O • Bicyclo(621)-oxa • Epoxidation, peracid • CompOx-Alkene/Alkene+Peracid • Epoxide → Diol • Ketone+Mg-Alkyl • Stereoconvergent • Mechanism • |
| Reagents: | Pd(OAc)2 • Cu(OAc)2 • CrO3, H2SO4 (Jones) • LiNiPr2 • I2 • InCl3 • BH4-Na+ • AlHiBu2 • Acetic anhydride • Sc(OTf)3 • Cyanide, trimethylsilyl • Alkyl-MgX • Grubbs II • Perbenzoic acid, 3-chloro • Hydroxide, lithium • Bisulfate, potassium • Sc(OTf)3 • Me-MgCl • |