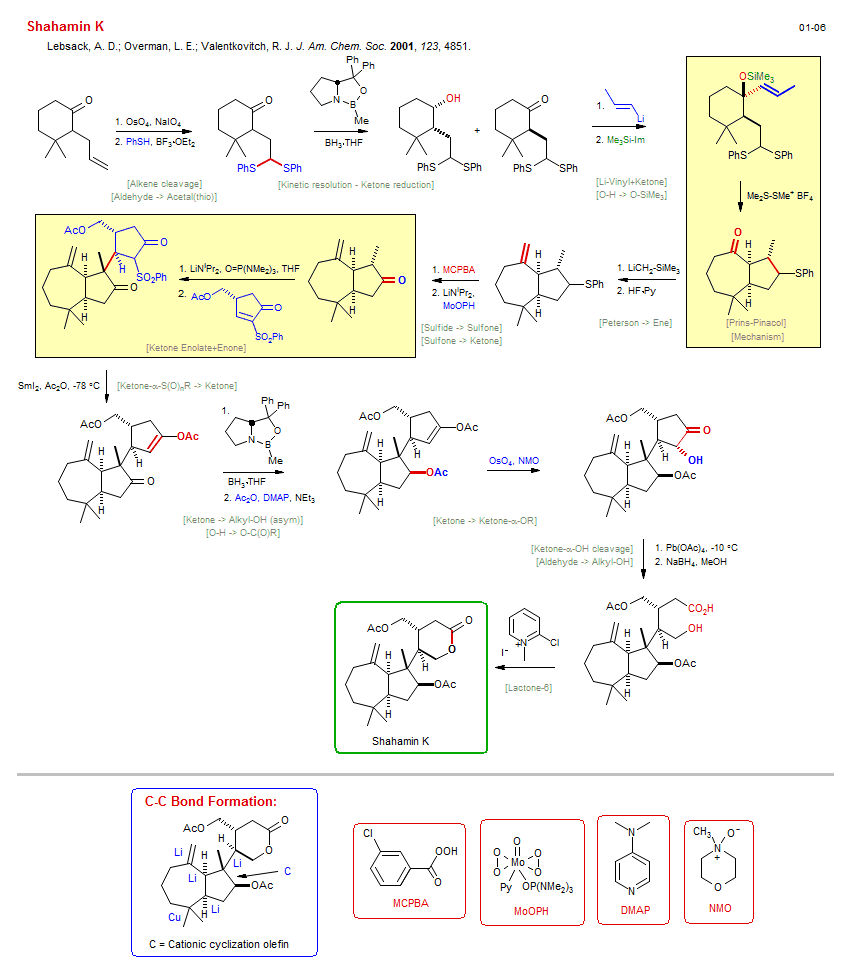
Total synthesis of Shahamin K:
| Reference: | Lebsack, A. D.; Overman, L. E.; Valentkovitch, R. J. J. Am. Chem. Soc. 2001, 123, 4851. DOI |
| Reactions: | Ketone → Alkyl-OH (asym) • Kinetic resolution - Ketone reduction • Peterson → Ene • Li-Silane+Ketone • Li-Vinyl+Ketone • Sulfone → Ketone • Aldehyde → Acetal(thio) • Ketone → Ketone-α-OR • CompOx-Alkene/Alkene+OsO4 • Lactone-6 • Prins-Pinacol • Cyclopentane • Bicyclo(530) • Mechanism • Ketone Enolate+Enone • ConjAdd Enolate • Ketone → Alkyl-OH (asym) • Alkene cleavage • Lemieux-Johnson oxidation • O-H → O-SiMe3 • Sulfide → Sulfone • Ketone-α-S(O)nR → Ketone • O-H → O-C(O)R • Ketone-α-OH cleavage • Criegee oxidation • Aldehyde → Alkyl-OH • |
| Reagents: | OsO4, NaIO4 • Thiol, phenyl • Chiral catalyst (amino-alcohol) • Corey oxazaborolidine • BH3·THF • Propenyl-Li • SiMe3-Im • LiCH2-SiR3 • HF·Py • Perbenzoic acid, 3-chloro • LiNiPr2 • SmI2 • Chiral catalyst (amino-alcohol) • Corey oxazaborolidine • BH3·THF • Acetic anhydride, DMAP • OsO4, NMO • Pb(OAc)4 • BH4-Na+ • Pyridinium, 2-halo • MoOPH • BF3·OEt2 • Acetic anhydride • Me2S-SMe+ X- • LiNiPr2, HMPA • |