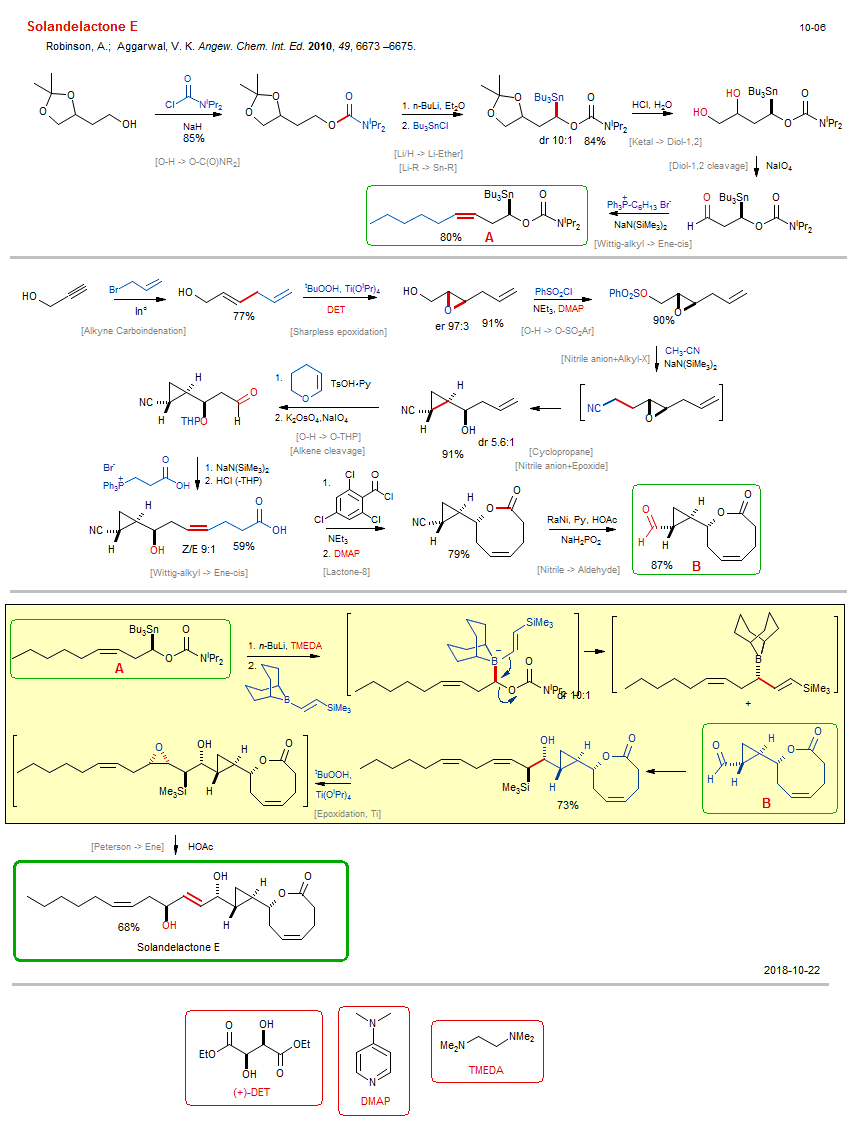
Total synthesis of Solandelactone E:
| Reference: | Robinson, A.; Aggarwal, V. K. Angew. Chem. Int. Ed. 2010, 49, 6673 –6675. DOI |
| Reactions: | Sharpless epoxidation • O-H → O-C(O)NR2 • Li/H → Li-Ether • Li-R → Sn-R • Ketal → Diol-1,2 • Diol-1,2 cleavage • Wittig-alkyl → Ene-cis • Alkyne Carboindenation • O-H → O-SO2Ar • Nitrile anion+Alkyl-X • Lactone-8 • Yamaguchi lactonization • Nitrile → Aldehyde • Wittig-alkyl → Ene-cis • Epoxidation, Ti • Peterson → Ene • Cyclopropane • Alkene cleavage • Nitrile anion+Epoxide • Epoxide+Nitrile anion (intram) • O-H → O-THP • |
| Reagents: | O=C(NR2)Cl • NaH • BuLi(n) • SnBu3-Cl • HCl, H2O • Periodate, sodium • PPh3=CH-Alkyl • NaN(SiMe3)2 • Allyl-Br • Hydroperoxide, tbutyl - Ti • Tartrate, diethyl • Epoxide • Sulfonyl chloride, benzene • NEt3 • DMAP • Acetonitrile (react) • NaN(SiMe3)2 • OsO4-22K+ • PPh3=CH-Alkyl • NaN(SiMe3)2 • Benzoyl chloride, 2,4,6-trichloro • NEt3 • DMAP • RaNi • BuLi(n) • Allyl-BR2 • Vinyl-SiR3 • Hydroperoxide, tbutyl - Ti • Acetic acid • TMEDA • DHP • TsOH·Py • |