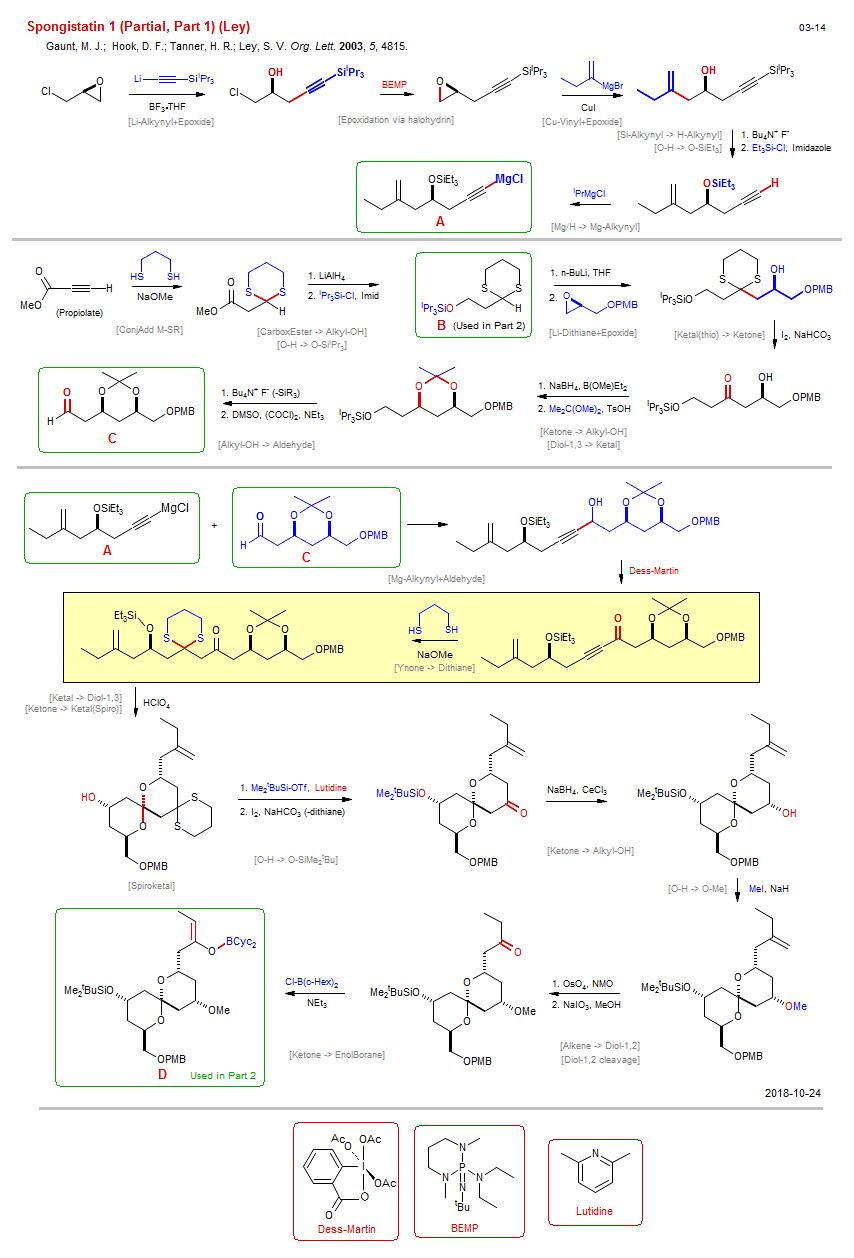
Total synthesis of Spongistatin 1 (Partial, Part 1) (Ley):
| Reference: | Gaunt, M. J.; Hook, D. F.; Tanner, H. R.;Ley, S. V. Org. Lett. 2003, 5, 4815. DOI (Part 2) (Part 3) (Part 4) |
| Reactions: | Li/H → Li-Dithiane • Diol-1,3 → Ketal • O-SiiPr3 → O-H • Propargyl-OH → Ynone • Li-Dithiane+Epoxide • Li-Alkynyl+Epoxide • Epoxide+Li-R • Li/H → Li-Alkynyl • Cu-Vinyl+Epoxide • Epoxide+Cu-R • Mg-R → Cu-R • Mg-Alkynyl+Aldehyde • Ynone → Dithiane • ConjAdd M-SR • Ynone+Thiolate • Diol-1,3 → Ketal • Epoxidation via halohydrin • Chlorohydrin → Epoxide • Ketone → Alkyl-OH • Felkin-Anh Chelation • ConjAdd M-SR • Ynoate → Dithiane • YnoateEster+Thiolate • CarboxEster → Alkyl-OH • O-H → O-SiiPr3 • Ketal(thio) → Ketone • Alkyl-OH → Aldehyde • Mg/H → Mg-Alkynyl • Si-Alkynyl → H-Alkynyl • O-H → O-SiEt3 • Ketal(thio) → Ketone • Ketone → Ketal(Spiro) • Ketal → Diol-1,3 • Spiroketal • O-H → O-SiMe2tBu • Ketone → Alkyl-OH • O-H → O-Me • Alkene → Diol-1,2 • Diol-1,2 cleavage • Alkene cleavage • Ketone → EnolBorane • |
| Reagents: | Epoxide • Alkynyl-Li • BF3·THF • Alkynyl-SiR3 • Epoxide • Vinyl-MgX • CuI • Fluoride, R4N+ • SiEt3-Cl • BuLi(n) • Epoxide • I2, NaHCO3 • BH4-Na+ • Me2C(OMe)2 • Fluoride, R4N+ • DMSO, (COCl)2 (Swern) • Alkyl-MgX • Dess-Martin • Thiol, (CH2)3-SH • AlH4-Li+ • SiiPr3-Cl • Propiolate • Imidazole • Alkynyl-MgX • Alkynyl-MgX • Thiol, (CH2)3-SH • HClO4 • SiMe2tBu-OTf • I2, NaHCO3 • BH4-Na+, CeCl3 • MeI • OsO4, NMO • Iodide, sodium • NEt3 • Lutidine • |