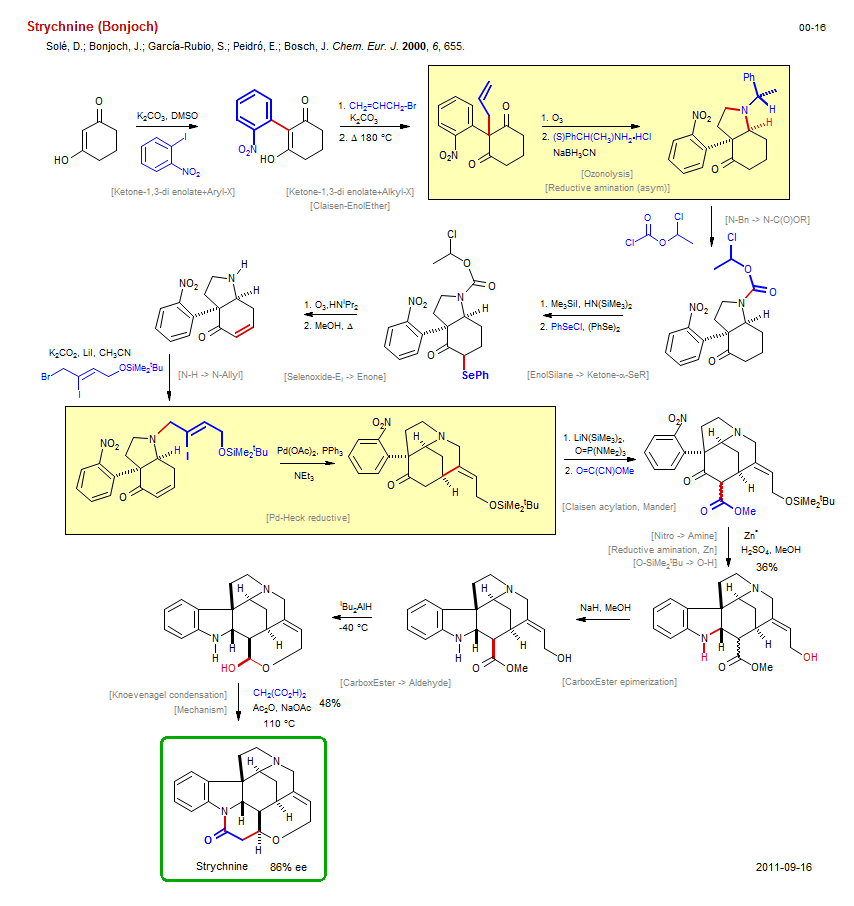
Total synthesis of Strychnine (Bonjoch):
| Reference: | Solé, D.; Bonjoch, J.; García-Rubio, S.; Peidró, E.; Bosch, J. Chem. Eur. J. 2000, 6, 655. DOI |
| Reactions: | Ketone → EnolSilane • N-C(O)OR → N-CH3 • Lactam-6 • Ketone-1,3-di enolate+Aryl-X • Ketone-1,3-di enolate+Alkyl-X • Allyl-X+Enolate • Reductive amination (asym) • Amine-5 • Bicyclo(430)-aza • N-Bn → N-C(O)OR • EnolSilane → Ketone-α-SeR • Selenoxide-Ei → Enone • N-H → N-Allyl • Pd-Heck reductive • Amine-6 • Claisen acylation, Mander • Ketone enolate+Mander • Mander+enolate • Nitro → Amine • Indole synthesis • CarboxEster epimerization • CarboxEster → Aldehyde • Mechanism • Knoevenagel condensation • Lactam-6 • Ether-7 • Claisen-EnolEther • Ozonolysis • O-SiMe2tBu → O-H • Reductive amination, Zn • |
| Reagents: | Carbonate, potassium • Allyl-Br • Carbonate, potassium • O3 • BH3CN-Na+ • NH(SiMe3)2 (HMDS) • SePh-Cl • O3 • Vinyl-I • Allyl-Br • Pd(OAc)2 • NEt3 • LiN(SiMe3)2 • HMPA (solvent) • O=C(OR)CN • H2SO4 • NaH • AlHiBu2 • Malonate • Acetate, sodium • NHiPr2 • Iodide, lithium • |