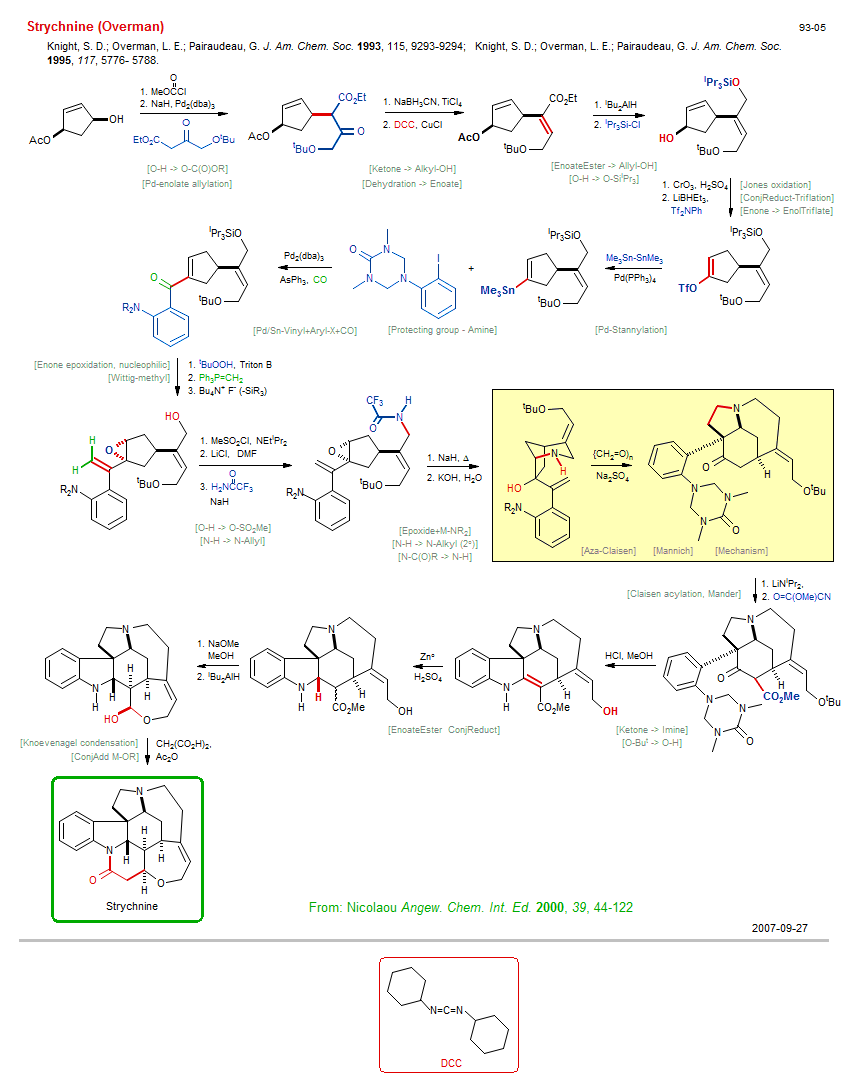
Total synthesis of Strychnine (Overman):
| Reference: | Knight, S. D.; Overman, L. E.; Pairaudeau, G. J. Am. Chem. Soc. 1993, 115, 9293-9294; DOI Knight, S. D.; Overman, L. E.; Pairaudeau, G. J. Am. Chem. Soc. 1995, 117, 5776- 5788. DOI |
| Reactions: | Protecting group - Amine • O-SiiPr3 → O-H • Alkyl-X → Alkyl-Cl • Finkelstein reaction • CarboxEster → Aldehyde • Ether-7 • Lactam-6 • Knoevenagel condensation • Mechanism • ConjAdd M-OR • Ether-7 • Ketone → Imine • Amine-5 • Aminal → Amine • EnoateEster ConjReduct • N-H → N-Allyl • N-H → N-Alkyl (2°) • Pd/Sn-Vinyl+Aryl-X+CO • Stille carbonylative coupling • Carbonylation • Pd-enolate allylation • Tsuji-Trost allylation • CompE+-Allyl-X/Allyl-X+Pd-enolate • Enone → EnolTriflate • ConjReduct-Triflation • Enone ConjReduct • Pd-Stannylation • EnolTriflate → Sn-Vinyl • Wittig-methyl • Enone epoxidation, nucleophilic • CompOx-Enone/Alkene+ROOH • Dehydration → Enoate • Claisen acylation, Mander • Ketone enolate+Mander • Mander+enolate • Mechanism • Mannich • Aza-Claisen • Amine-5 • Bicyclo(430)-aza • Ketone → Alkyl-OH • CompRed-Ketone/CarboxEster+M-H • O-H → O-C(O)OR • EnoateEster → Allyl-OH • Epoxide+M-NR2 • Amine-6 • Bicyclo(321)-aza • N-C(O)R → N-H • Jones oxidation • Allyl-OH → Enone • O-H → O-SiiPr3 • O-H → O-SO2Me • O-But → O-H • |
| Reagents: | O=C(OR)Cl • NaH • Pd2(dba)3 • Acetoacetate • BH3CN-Na+ • TiCl4 • Carbodiimide • CuCl • AlHiBu2 • SiiPr3-Cl • CrO3, H2SO4 (Jones) • BH(Et)3-Li+ • Tf2NPh • SnMe3-SnMe3 • Pd(PPh3)4 • Pd2(dba)3 • AsPh3 • CO • Hydroperoxide, tbutyl • Triton B • PPh3=CH2 • Fluoride, R4N+ • Sulfonyl chloride, methane • NEtiPr2 • Chloride, lithium • Acetamide, trifluoro • NaH • Epoxide • NaH • Hydroxide, potassium • LiNiPr2 • O=C(OR)CN • HCl, MeOH • Zn° • H2SO4 • NaOMe • AlHiBu2 • Malonate • Acetic anhydride • DMF (solvent) • Formaldehyde • Sulfide, sodium • |