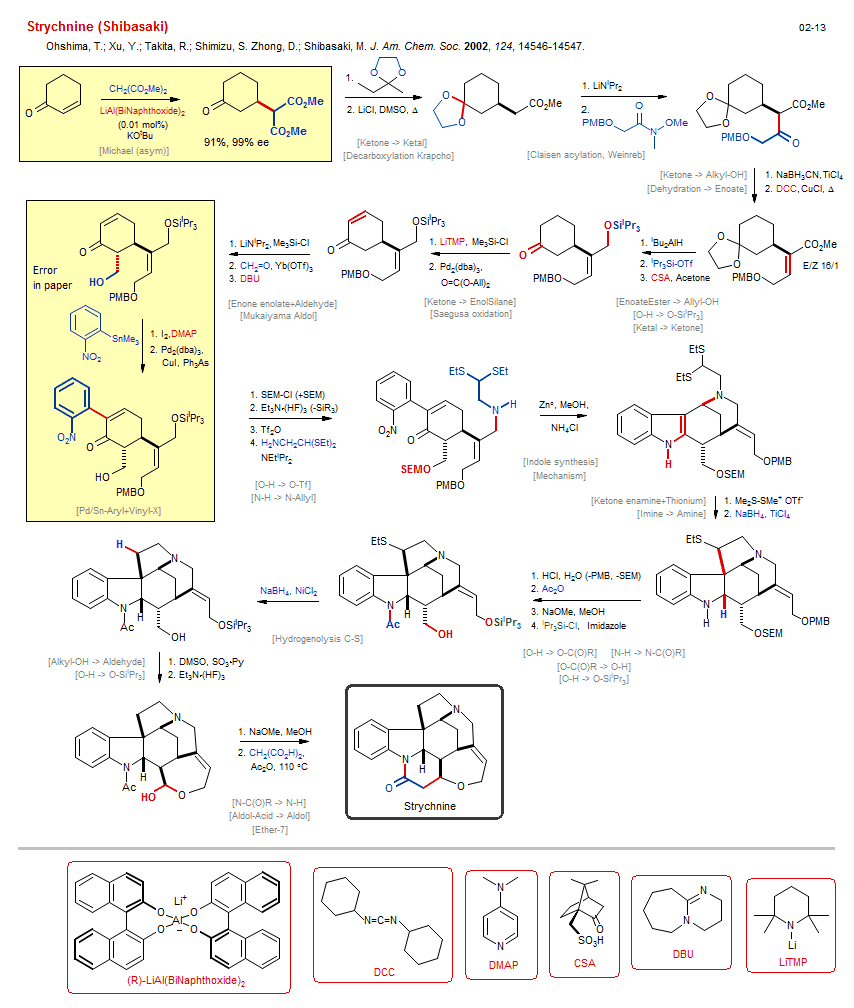
Total synthesis of Strychnine (Shibasaki):
| Reference: | Ohshima, T.; Xu, Y.; Takita, R.; Shimizu, S. Zhong, D.; Shibasaki, M. J. Am. Chem. Soc. 2002, 124, 14546-14547. DOI |
| Reactions: | Ketone epimerization • O-H → O-SEM • O-SiiPr3 → O-H • O-PMB → O-H • O-SEM → O-H • Claisen acylation, Weinreb • CarboxEster enolate+CarboxAmide • CarboxAmide(Weinreb)+Enolate • Enone enolate+Aldehyde • Saegusa oxidation • Ketone → Enone • Decarboxylation Krapcho • N-H → N-Allyl • Hydrogenolysis C-S • Alkyl-SR → Alkyl-H • Ketone enamine+Thionium • Amine-5 • Bicyclo(430) • Indole synthesis • Amine-6 • Bicyclo(331)-aza • Mechanism • Amine-6 • Mukaiyama Aldol • Aldol-Si • Aldehyde+EnolSilane • Dehydration → Enoate • Michael (asym) • CarboxEster enolate+Enone • ConjAdd Enolate • Enone → Enone-α-Halo • Pd/Sn-Aryl+Vinyl-X • Ether-7 • Lactam-6 • Ketone → Ketal • Ketone → Alkyl-OH • CompRed-Ketone/CarboxEster+M-H • Ketone → EnolSilane • O-H → O-SiiPr3 • Ketal → Ketone • O-H → O-Tf • Imine → Amine • Alkyl-OH → Aldehyde • CompOx-Alkyl-OH/Amine+DMSO • O-H → O-SiiPr3 • N-C(O)R → N-H • Aldol-Acid → Aldol • Ether-7 • Lactam-6 • O-H → O-C(O)R • N-H → N-C(O)R • O-C(O)R → O-H • O-H → O-SiiPr3 • |
| Reagents: | Chloride, lithium • LiNiPr2 • Weinreb acetamide • BH3CN-Na+ • Carbodiimide • AlHiBu2 • SiiPr3-OTf • Sulfonic acid, Camphor • LiTMP • Pd2(dba)3 • LiNiPr2 • Formaldehyde • DBU • SEM-Cl • HF·NEt3 • Tf2O • Chloride, ammonium • Me2S-SMe+ X- • BH4-Na+, TiCl4 • HCl, H2O • DMSO, Py·SO3 (Parikh-Doering) • NaOMe • Malonate • HF·NEt3 • Acetic anhydride • Yb(OTf)3 • NaOMe • O=C(OR)2 • CuCl • BH4-Na+, NiCl2 • NEtiPr2 • TiCl4 • SiMe3-Cl • SiMe3-Cl • Acetic anhydride • SiiPr3-Cl • Imidazole • DMSO (solvent) • Malonate • Chiral catalyst (diol) • KOtBu • I2 • AsPh3 • Aryl-SnR3 • DMAP • HO-Allyl • Al(OR)4Li • |