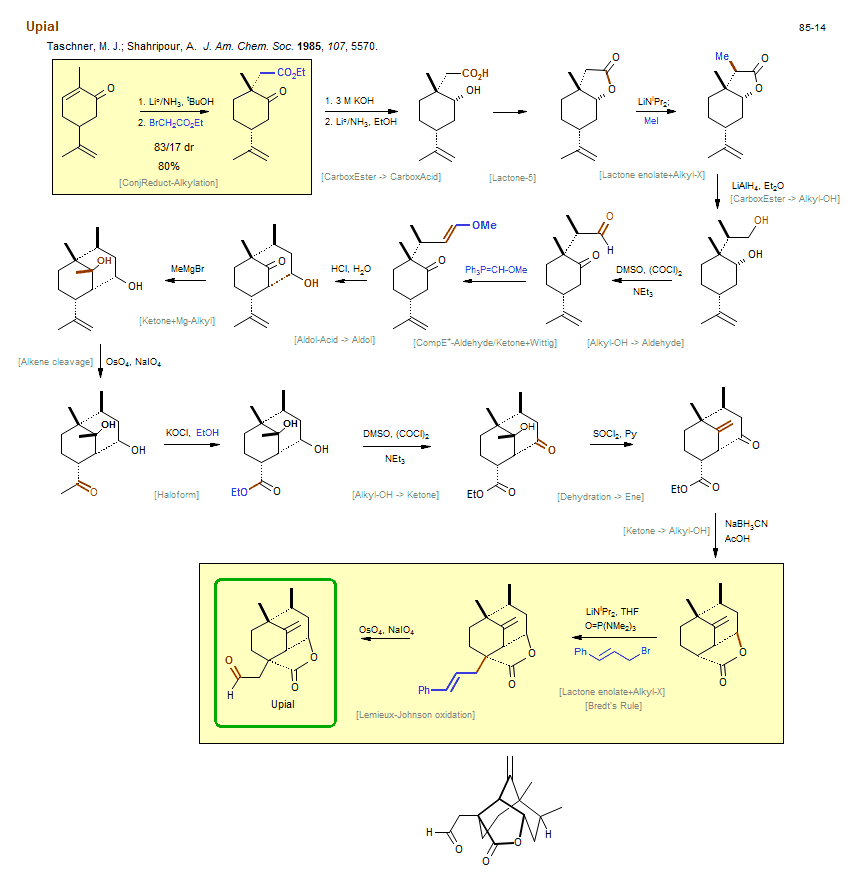
Total synthesis of Upial:
| Reference: | Taschner, M. J.; Shahripour, A. J. Am. Chem. Soc. 1985, 107, 5570. DOI |
| Reactions: | ConjReduct-Alkylation • Ketone enolate+Alkyl-X • Bromoacetate+Enolate • Enone ConjReduct • Lactone enolate+Alkyl-X • Lactone-5 • Alkyl-X+Enolate • CarboxEster → CarboxAcid • Lactone-5 • Wittig-methoxymethyl • Reductive formylation • Ketone+Mg-Alkyl • Mg-Alkyl+Ketone • Aldol-Acid → Aldol • Cyclohexanone • Bicyclo(331) • CompE+-Aldehyde/Ketone+Wittig • CarboxEster → Alkyl-OH • Alkyl-OH → Aldehyde • Haloform • Lactone-5 • Bredt's Rule • Lactone enolate+Alkyl-X • Allyl-X+Enolate • Dehydration → Ene • Alkene cleavage • Lemieux-Johnson oxidation • Ketone → Alkyl-OH • CompRed-Ketone/CarboxEster+M-H • Lactone-5 • Alkyl-OH → Ketone • Lemieux-Johnson oxidation • Alkene cleavage • |
| Reagents: | Li°/NH3 • Bromoacetate • Hydroxide, potassium • Li°/NH3 • LiNiPr2 • MeI • AlH4-Li+ • DMSO, (COCl)2 (Swern) • NEt3 • PPh3=CH-OR • HCl, H2O • Me-MgBr • OsO4, NaIO4 • SOCl2 • BH3CN-Na+ • Acetic acid • LiNiPr2 • Allyl-Br • OsO4, NaIO4 • Hypochlorite, potassium • DMSO, (COCl)2 (Swern) • HMPA (solvent) • HOEt (react)} • NEt3 • |