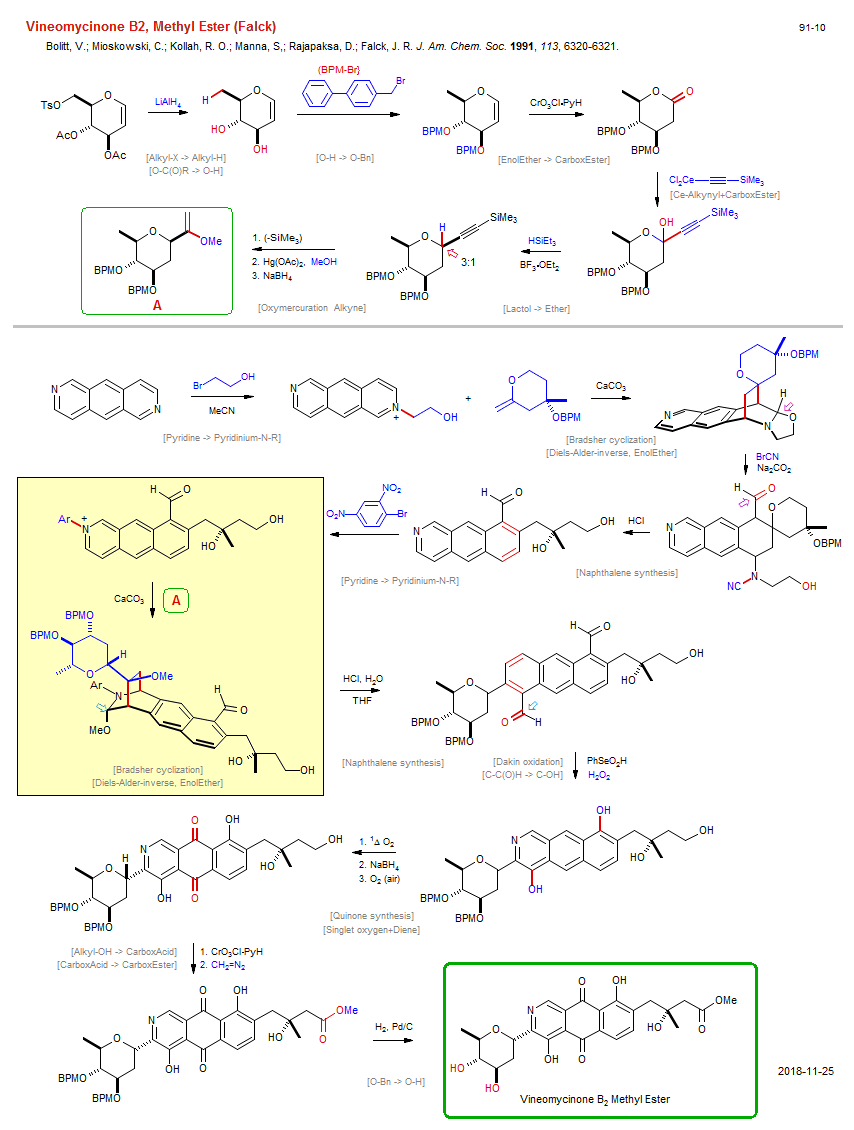
Total synthesis of Vineomycinone B2, Methyl Ester (Falck):
| Reference: | Bolitt, V.; Mioskowski, C.; Kollah, R. O.; Manna, S,; Rajapaksa, D.; Falck, J. R. J. Am. Chem. Soc. 1991, 113, 6320-6321. DOI |
| Reactions: | Bradsher cyclization • Bradsher cyclization • Dakin oxidation • Baeyer-Villiger • Alkyl-X → Alkyl-H • O-C(O)R → O-H • O-H → O-Bn • EnolEther → CarboxEster • Ce-Alkynyl+CarboxEster • Lactol → Ether • Oxymercuration Alkyne • Pyridine → Pyridinium-N-R • Pyridine → Pyridinium-N-R • Diels-Alder-inverse, EnolEther • Diels-Alder-inverse, EnolEther • Naphthalene synthesis • C-C(O)H → C-OH • Quinone synthesis • Singlet oxygen+Diene • Alkyl-OH → CarboxAcid • CarboxAcid → CarboxEster • O-Bn → O-H • Naphthalene synthesis • |
| Reagents: | AlH4-Li+ • CrO3Cl·PyH (PCC) • Alkynyl-CeX2 • Alkynyl-SiR3 • HSiEt3 • BF3·OEt2 • Hg(OAc)2 • HOMe (react) • BH4-Na+ • Carbonate, calcium • Bromoethanol • Acetonitrile (solvent) • HCl • Carbonate, calcium • HCl, H2O • SeO2 • H2O2 • O2 (singlet) • BH4-Na+ • CrO3Cl·PyH (PCC) • Diazomethane • H2, Pd • |