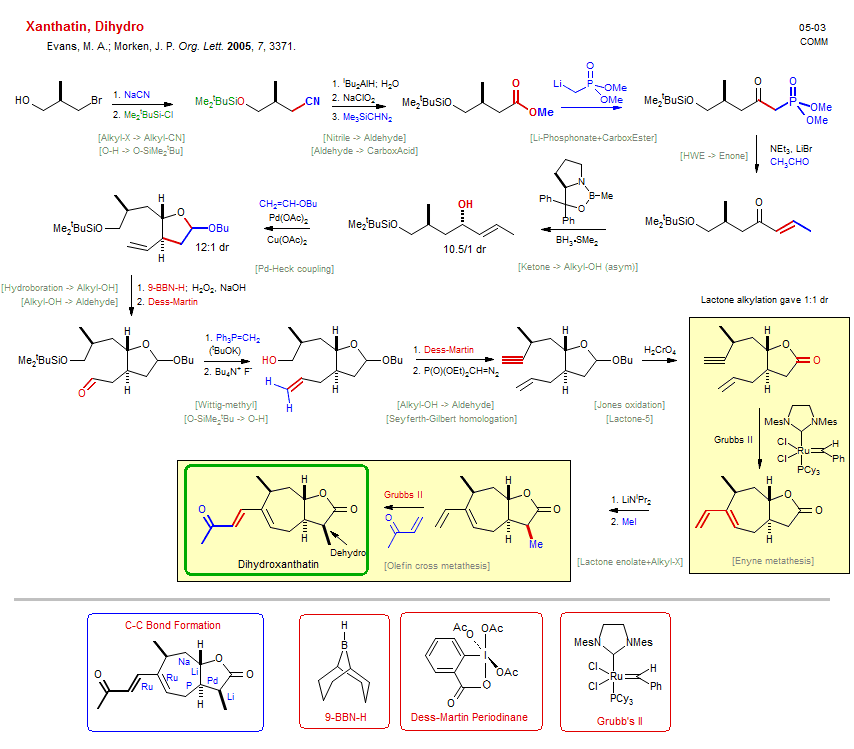
Total synthesis of Xanthatin, Dihydro:
| Reference: | Evans, M. A.; Morken, J. P. Org. Lett. 2005, 7, 3371. DOI |
| Reactions: | CarboxAcid → CarboxEster • Enone → Allyl-OH (asym) • HWE → Enone • Li-Phosphonate+CarboxEster • Ketone → Alkyl-OH (asym) • Wittig-methyl • Seyferth-Gilbert homologation • Aldehyde → Alkyne • Enyne metathesis • Cycloheptane • Olefin cross metathesis • Hydroboration → Alkyl-OH • Nitrile → Aldehyde • EnolEther → acetal • Alkyl-X → Alkyl-CN • Lactone enolate+Alkyl-X • Alkyl-X+Enolate • Pd-Heck coupling • Lactone-5 • Alkyl-OH → Aldehyde • O-SiMe2tBu → O-H • Alkyl-OH → Aldehyde • Jones oxidation • Lactol → Lactone • O-H → O-SiMe2tBu • Aldehyde → CarboxAcid • Pinnick oxidation • |
| Reagents: | Cyanide, sodium • SiMe2tBu-Cl • AlHiBu2 • Chlorite, sodium • Diazomethane, silyl • P(O)(OR)2CH2-C(O)R' • Bromide, lithium • Corey oxazaborolidine • Chiral catalyst (amino-alcohol) • BH3·SMe2 • Cu(OAc)2 • BH-BBN • Dess-Martin • PPh3=CH2 • KOtBu • Fluoride, R4N+ • Dess-Martin • CrO3, H2SO4 (Jones) • LiNiPr2 • MeI • Grubbs II • Methyl vinyl ketone • Grubbs II • Vinyl-OR • Diazophosphonate • H2O2, NaOH • Acetaldehyde • Pd(OAc)2 • |