Research Description
Our research effort is directed towards the study of organometallic and organometalloid compounds. The goal of the work is to deepen our understanding of these compounds and thus improve and extend their chemistry, as well as to discover new reactions of value for synthetic chemistry.
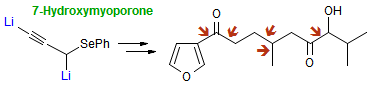
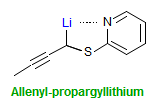
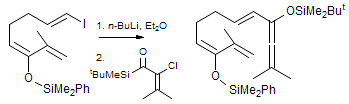
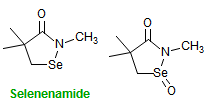
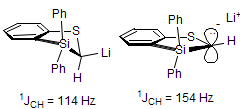
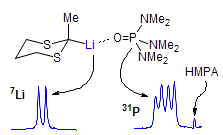
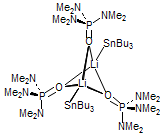
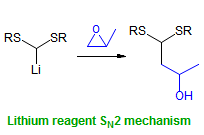
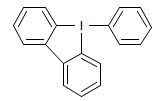
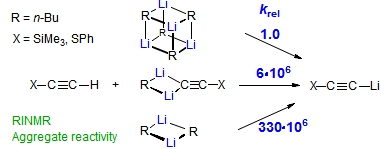
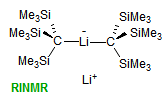
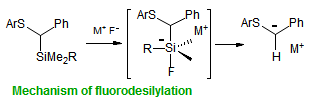
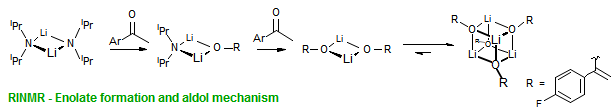
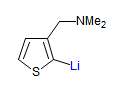
The majority of C-C bond forming reactions involve the interaction of carbanionic centers with electrophilic carbon species. Organolithium reagents are probably the single most important source of carbanionic species, and they have long played an important role in synthetic organic chemistry. A vast literature provides many recipes for preparing and utilizing them. However, the basis for much of what we do in the laboratory when we use lithium reagents is empirical, and is not based on firm mechanistic and structural insights. We are trying to understand in detail some reactions and properties of organolithium reagents. Coordinating solvents and co-solvents such as THF, TMEDA, HMPA, crown ethers and others play important roles for fine tuning the reactivity of organolithium reagents: stereochemical and regiochemical selectivity is profoundly affected by such additives, but we do not understand the origin of these changes. We have developed NMR spectroscopic tools for gaining detailed insights into the structures of lithium reagents in solution. When such studies are combined with reactivity and kinetic studies, we anticipate that organolithium reactions can be understood at the molecular level. This work has led to the discovery of new anionic complexes of organometalloids (e.g., Ph2I-Li+, Ph3Te-Li+, Me5Sn-Li+) which are intermediates in the metal-halogen exchange and transmetallation reactions, and which may behave as carbanion donors in their own right under some conditions.
In the course of an investigation of how organolithium reagents invert configuration we found that an important pathway involved organolithium aggregates. When appropriate solvent additives are used to convert the organolithium reagent to a monomer, then significant improvement in configurational stability results. Now even sec-alkylithiums (e.g.,Mcyclohexyllithiums), long thought to be configurationally stable only in hydrocarbon solvents, can be prepared with high configurational purity in solvents as polar as THF.
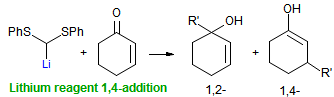
Other questions we are interested in are the following: What determines whether a 1-substituted allylmetal reacts at the α- or γ- position? What determines whether an organometallic reagent adds 1,2 or 1,4 to an α,β-unsaturated carbonyl compound? Why do lithium halides sometimes have such dramatic effects on organolithium reactions? What species are formed when organolithium species are transmetallated to organocerium, manganese, titanium and other organometallics?
| 1960 - 1964 | B. Sc. with Prof. Karl Kopecky at University of Alberta - Edmonton |
 | |
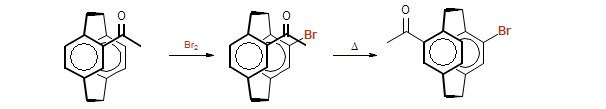
| 1964 - 1968 | Ph.D. with Prof. D. J. Cram, UCLA |
 | |
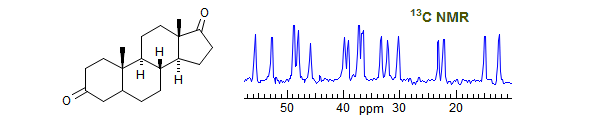
| 1968 - 1969 | Postdoc with Prof. J. D. Roberts, California Institute of Technology |
 | |
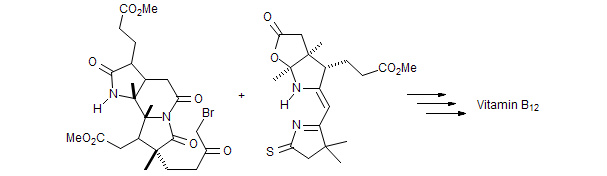
| 1969 - 1970 | Postdoc with Prof. R. B. Woodward, Harvard University |
 | |
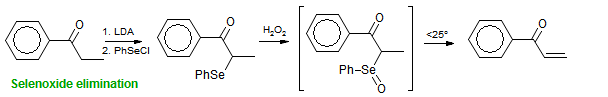
| 1970-2020 | Professor of Chemistry - University of Wisconsin, Madison |
  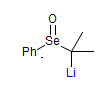 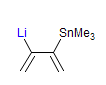 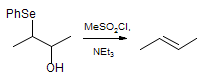     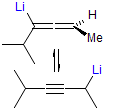   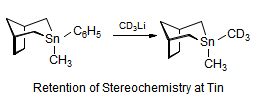   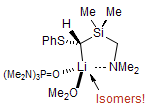    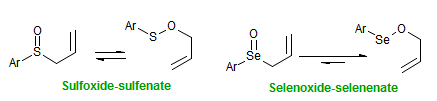  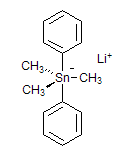  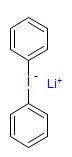 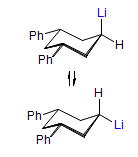 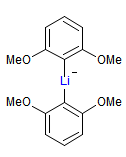  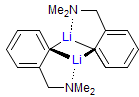 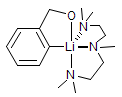       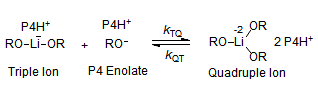  | |
| 1979 | Visiting Professor University in Marburg, Germany with Prof. Reinhard Hoffmann |
| 1987 | Visiting Professor University of Strasbourg, France with Prof. Francois Biellmann |
| 1997 | Visiting Professor University of Alicante, Spain with Prof. Miguel Yus |
| Professor Reich's full list of publications and website resources is available. | |